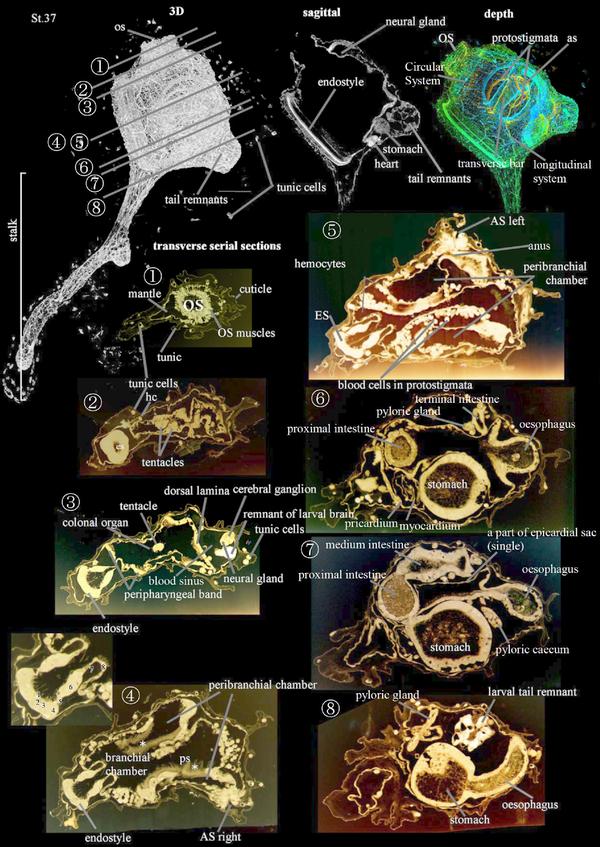
Stage 37
This is the Early Juvenile I Stage (CirobuD:0000060), which occurs when the endostyle axis is almost parallel to the axis passing through the stalk (63-72 h (3 dpf), Stage 37). The stomach swells and the larval tail remnants are no longer present 4 dpf.
The hermaphrodite reproductive system (CirobuA:0000909) is now recognizable. The female reproductive system (CirobuA:0000910) is formed by a sac-like ovary (CirobuA:0000672) continuous in an oviduct (CirobuA:0000801). The male reproductive system (CirobuA:0000910) comprises the lobular testis encrusting the ovary (CirobuA:0000742) and the sperm duct (CirobuA:0000920). Germ cells (CirobuA:0000916) are maturing within the gonads.
The stalk base forms test villi (CirobuA:0000927), each one furnished with a test vessel (CirobuA:0000928) in continuity with the haemocele. They ensure firm adhesion to the substrate. In the body, several blood sinuses (CirobuA:0000856) can be recognized among organs.
After Stage 37, other territories become histologically recognizable (data not shown). These are the cloacal cavity (CirobuA:0000852), the dorsal languets (CirobuA:0000636) on the roof of the branchial chamber (CirobuA:0000863), the pharyngo-epicardial openings (CirobuA:0000868) putting the epicardiac cavities (CirobuA:0000879) in communication with the branchial one, the endostylar appendix (CirobuA:0000866), the oral pigment spots (CirobuA:0000899) and the atrial pigment spots (CirobuA:0000854) encircling the oral and the cloacal siphon border, respectively.
Bostwick, M., Smith, E.L., Borba, C., Newman-Smith, E., Guleria, I., Kourakis, M.J., Smith, W.C., 2020. Antagonistic Inhibitory Circuits Integrate Visual and Gravitactic Behaviors. Curr. Biol. 1–10. https://doi.org/10.1016/j.cub.2019.12.017
Burighel, P., Cloney, R.A., Cloney, B., 1997. Microscopic Anatomy of Invertebrates, Vol. 15. Microsc. Anat. Invertebr. 15, 221–347.
Chiba, S., Sasaki, A., Nakayama, A., Takamura, K., Satoh, N., 2004. Development of Ciona intestinalis juveniles (through 2nd ascidian stage). Zoolog. Sci. 21, 285–298. https://doi.org/10.2108/zsj.21.285
Cloney, R.A., 1972. Cytoplasmic filaments and morphogenesis: effects of cytochalasin B on contractile epidermal cells. Zellforsch 132, 167–192.
Davidson, B., 2007. Ciona intestinalis as a model for cardiac development. Semin. Cell Dev. Biol. 18, 16–26. https://doi.org/10.1016/j.semcdb.2006.12.007
Davidson, B., Levine, M., 2003. Evolutionary origins of the vertebrate heart: Specification of the cardiac lineage in Ciona intestinalis. Proc. Natl. Acad. Sci. U. S. A. 100, 11469–73. https://doi.org/10.1073/pnas.1634991100
Hirano, T., Nishida, H., 2000. Developmental fates of larval tissues after metamorphosis in the ascidian, Halocynthia roretzi. II. Origin of endodermal tissues of the juvenile. Dev. Genes Evol. 210, 55–63. https://doi.org/10.1007/s004270050011
Hirano, T., Nishida, H., 1997. Developmental Fates of Larval Tissues after Metamorphosis in AscidianHalocynthia roretzi. Dev. Biol. 192, 199–210. https://doi.org/10.1006/dbio.1997.8772
Horie, T., Horie, R., Chen, K., Cao, C., Nakagawa, M., Kusakabe, T.G., Satoh, N., Sasakura, Y., Levine, M., 2018. Regulatory cocktail for dopaminergic neurons in a protovertebrate identified by whole-embryo single-cell transcriptomics. Genes Dev. 32, 1297–1302. https://doi.org/10.1101/gad.317669.118
Hozumi, A., Horie, T., Sasakura, Y., 2015. Neuronal map reveals the highly regionalized pattern of the juvenile central nervous system of the ascidian Ciona intestinalis. Dev. Dyn. 244, 1375–1393. https://doi.org/10.1002/dvdy.24317
Imai, J.H., Meinertzhagen, I.A., 2007. Neurons of the Ascidian Larval Nervous System in Ciona intestinalis: I. Central Nervous System. J. Comp. Neurol. 501, 316–334. https://doi.org/10.1002/cne
Karaiskou, A., Swalla, B.J., Sasakura, Y., Chambon, J.-P.P., 2015. Metamorphosis in solitary ascidians. Genesis 53, 34–47. https://doi.org/10.1002/dvg.22824
Kawamura, K., Tiozzo, S., Manni, L., Sunanaga, T., Burighel, P., De Tomaso, A.W., 2011. Germline cell formation and gonad regeneration in solitary and colonial ascidians. Dev. Dyn. 240, 299–308. https://doi.org/10.1002/dvdy.22542
Lash, J.W., Cloney, R.A., Minor, R.R., 1973. The effect of cytochalasin B upon tail resorption and metamorphosis in ten species of ascidians. Biol. Bull. 145, 360–72. https://doi.org/10.2307/1540046
Mackie, G.O., Burighel, P., Caicci, F., Manni, L., 2006. Innervation of ascidian siphons and their responses to stimulation. Can. J. Zool. 84, 1146–1162. https://doi.org/10.1139/z06-106
Manni, L., Agnoletto, A., Zaniolo, G., Burighel, P., 2005. Stomodeal and neurohypophysial placodes in Ciona Intestinalis: insights into the origin of the pituitary gland. J. Exp. Zool. Part B Mol. Dev. Evol. 304B, 324–339. https://doi.org/10.1002/jez.b.21039
Matsunobu, S., Sasakura, Y., 2015. Time course for tail regression during metamorphosis of the ascidian Ciona intestinalis. Dev. Biol. 405, 71–81. https://doi.org/10.1016/j.ydbio.2015.06.016
Moret, F., Christiaen, L., Deyts, C., Blin, M., Joly, J.S., Vernier, P., 2005. The dopamine-synthesizing cells in the swimming larva of the tunicate Ciona intestinalis are located only in the hypothalamus-related domain of the sensory vesicle. Eur. J. Neurosci. 21, 3043–3055. https://doi.org/10.1111/j.1460-9568.2005.04147.x
Nakazawa, K., Yamazawa, T., Moriyama, Y., Ogura, Y., Kawai, N., Sasakura, Y., Saiga, H., 2013. Formation of the digestive tract in Ciona intestinalis includes two distinct morphogenic processes between its anterior and posterior parts. Dev. Dyn. 242, 1172–83. https://doi.org/10.1002/dvdy.24009
Numakunai, T., 1977. Hentai, in: Japan, T.Z.S. of (Ed.), Gendai Doubutsugaku No Kadai Vol.5. Gakkai Shuppan Center, pp. 135–175.
Ogasawara, M., Satoh, N., 1998. Isolation and Characterization of Endostyle-Specific Genes in the Ascidian Ciona intestinalis. Biol. Bull. 195, 60–69. https://doi.org/10.2307/1542776
Parrinello, D., Parisi, M., Parrinello, N., Cammarata, M., 2020. Ciona robusta hemocyte populational dynamics and PO-dependent cytotoxic activity. Dev. Comp. Immunol. 103, 103519. https://doi.org/10.1016/j.dci.2019.103519
Razy-Krajka, F., Brown, E.R., Horie, T., Callebert, J., Sasakura, Y., Joly, J.S., Kusakabe, T.G., Vernier, P., 2012. Monoaminergic modulation of photoreception in ascidian: evidence for a proto-hypothalamo-retinal territory. BMC Biol 10, 45. https://doi.org/10.1186/1741-7007-10-45
Ryan, K., Lu, Z., Meinertzhagen, I.A., 2016. The CNS connectome of a tadpole larva of Ciona intestinalis (L.) highlights sidedness in the brain of a chordate sibling. Elife 5, 1–34. https://doi.org/10.7554/eLife.16962
Shirae-Kurabayashi, M., Nishikata, T., Takamura, K., Tanaka, K.J., Nakamoto, C., Nakamura, A., 2006. Dynamic redistribution of vasa homolog and exclusion of somatic cell determinants during germ cell specification in Ciona intestinalis. Development 133, 2683–93. https://doi.org/10.1242/dev.02446
Stolfi, A., Gainous, T.B., Young, J.J., Mori, A., Levine, M., Christiaen, L., 2010. Early Chordate Origins of the Vertebrate Second Heart Field. Science (80-. ). 329, 565. https://doi.org/10.1126/science.202625
Stolfi, A., Lowe, E.K., Racioppi, C., Ristoratore, F., Brown, C.T., Swalla, B.J., Christiaen, L., 2014. Divergent mechanisms regulate conserved cardiopharyngeal development and gene expression in distantly related ascidians. Elife 3, e03728. https://doi.org/10.7554/eLife.03728
Terakubo, H.Q., Nakajima, Y., Sasakura, Y., Horie, T., Konno, A., Takahashi, H., Inaba, K., Hotta, K., Oka, K., 2010. Network structure of projections extending from peripheral neurons in the tunic of ascidian larva. Dev. Dyn. 239, 2278–87. https://doi.org/10.1002/dvdy.22361
Veeman, M.T., Newman-Smith, E., El-Nachef, D., Smith, W.C., 2010. The ascidian mouth opening is derived from the anterior neuropore: reassessing the mouth/neural tube relationship in chordate evolution. Dev. Biol. 344, 138–49. https://doi.org/10.1016/j.ydbio.2010.04.028
Wang, W., Razy-Krajka, F., Siu, E., Ketcham, A., Christiaen, L., 2013. NK4 Antagonizes Tbx1/10 to Promote Cardiac versus Pharyngeal Muscle Fate in the Ascidian Second Heart Field. PLoS Biol. 11, e1001725. https://doi.org/10.1371/journal.pbio.1001725