Reference:
A web-based interactive developmental table for the ascidian Ciona intestinalis, including 3D real-image embryo reconstructions: I. From fertilized egg to hatching larva. Dev Dyn. 2007 Jun 7;236(7):1790-1805 |
| Table 1. Stages of Early Embryonic Development in Ciona Intestinalis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
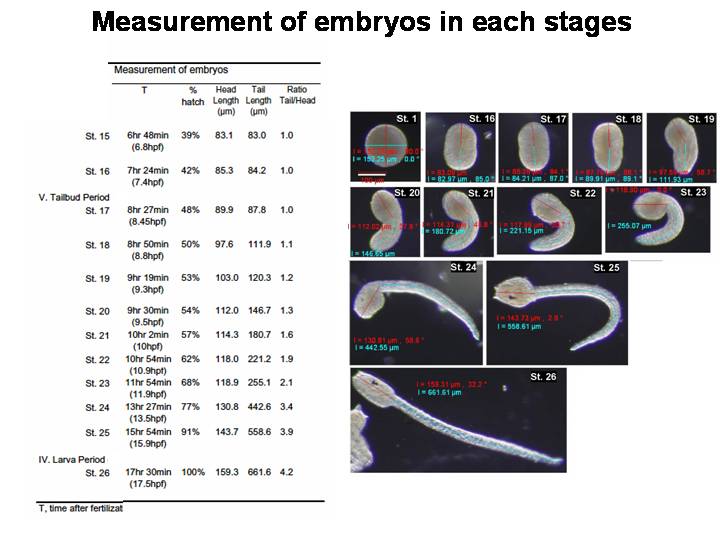
| From the left column, Hotta's stage is the staging criteria defined in this manuscript. A total of 26
stages are divided into six periods. Parentheses in each period mean
the start-time and end-time of each period at 18 degree centigrade. Characteristics is mainly based on the observation under dissecting microscopy. Measurement of embryos:
T, Time after fertilization (average at 18 degree centigrade, n=3), % hatch = rate of T
(min) / 1050 (min), head length, tail length and ratio of tail / head
length. |
We present this web-based image resource as the Four-dimensional Ascidian Body Atlas FABA (http://chordate.bpni.bio.keio.ac.jp/faba/top.html), which we believe will be advantageous not only for researchers who are interested in ascidian development but also for a general understanding of embryonic organization at the single cell level.
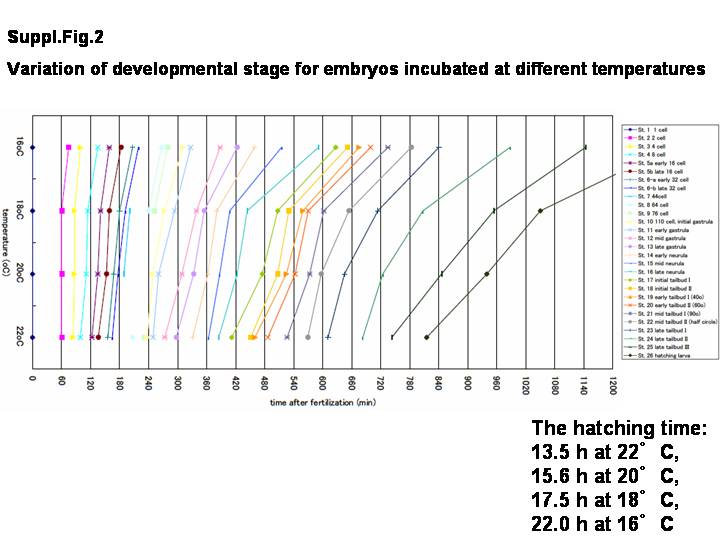
As the developmental time at one stage varies across different temperatures (see Supplemental Fig. 1, which ca be viewed at Suppl.Fig.1), another simple staging criteria that is not influenced by temperature but rather is transposable and based solely on morphology is required. Therefore, we introduce development progress here as % hatched, defined as the ratio percentage of time post-fertilization when all embryos have reached the hatching stage (where 100% hatched is reached in 17.5 hr at a fixed temperature of 18 degree centigrade). These new staging criteria are now defined and directly linked with each image (Table 1, Supplemental Fig. 2). Under these criteria, the spatio-temporal developmental patterns of embryos are accurately represented with the real-image reconstructed 3D whole embryos along with the normalized point in development progress.
Time-lapse images were acquired every 3 min post-fertilization at 18 degree centigrade (below). The fertilization occurred simultaneously and the cleavages were uniformly synchronized (data not shown). At 18 degree centigrade, chorionated embryos hatch into developing larvae at 17.5 hr post-fertilization (Supplemental Movie 1). Fixed embryos were then obtained, including cleavage, gastrulation, neurulation, formation of tailbud stage embryos, and the hatching larva. Based on newly defined staging criteria (Table 1), a total of 26 distinct representative embryos were chosen for imaging by CLSM. Images that showed a little deviation in developmental stages compared with those of majority of embryos were not collected. For a given developmental stage, we collected the majority as the representative embryo.

Alexa 546 phalloidine was used to visualize the position of cell membranes by staining cortical actin filaments. The fluorescent intensity of cytosolic actin staining is different among cells and embryonic stages (for example, see Fig. 4H). Therefore, this difference was useful for distinguishing the cell types in the developing embryos. All 3D images are reconstructed from the corresponding 100 sectioning images (LSM image browser, Zeiss, Germany).
To facilitate the visualization of both cross-sectional images and 3D images, these images were exported as a series of jpeg files, and they were converted into Flash (Adobe, San Jose, CA) files. The Flash plug-in can be easily installed into any web-browser. Each flash file includes image information on the spatio-temporal cell arrangement and was connected under the web-based database with the information of developmental stage, such as new developmental nomenclature, hour post-fertilization (hpf), % hatch, cell lineage, and scale (Figs. 1C, 3). These Flash files were integrated into the web-based database FABA, so that one can refer to every 15° angle of the 3D images along with the Y-axis interactively and to every z-section image via the internet ( http://chordate.bpni.bio.keio.ac.jp/faba/top.html).
We newly defined 26 stages for the embryonic and larval development of ascidian, Ciona intestinalis, in Table 1. These developmental stages began with fertilization and ended with hatching out from the chorion. Moreover, the numbering of the staging system was based on the staging system used for zebrafish and medaka (Kimmel et al., [1995]; Iwamatsu, [2004]). Table 1 also shows the time point relative to the developmental stages at 18 degree centigrade, and a new criteria described the important features of embryo, %hatch, and the embryonic size (below, i.e., the lengths of tail and head). Though embryos are dechorionated, hatching time was examined by co-incubation with chorionated embryos. Embryonic development was subdivided into six periods (Table 1): zygote, cleavage, gastrula, neurula, tailbud, and larva.
Until stage 10, the staging criteria depend both on the shape as well as the number of cells. After stage 10, to give stage names that are clearly understandable, criteria are mainly based on the shape and length, which are observed through a dissecting microscope under low magnification (Supplemental Fig. 1). These criteria are simple but provide suitable landmarks. For example, as a landmark of stage 20, an angular tail bend of 60° is selected. This tail bend is also an indicator of neuropore closure. At stage 21, the notochord intercalation finished. This stage landmark is identified by a 90° tail bend (or tail 1 ½ times longer than the trunk, Table 1).
Staging information is converted to standard developmental time, as designated by the letter h, and defined as normalized hours fertilization at 18°C. Previously reported optimal temperatures of incubation for C. intestinalis staging has varied from 16 to 20 degree centigrade (16 degree, Nicol and Meinertzhagen, [1988]; 18 degree, Whittaker, [1977]; 18-20 degree, Conklin, [1905]; 20 degree C, Chiba et al., [2004]). This difference in developmental rate between other temperatures is shown in Supplemental Figure 2 . The hatching time after fertilization consequently varied from 13.5 hr at 22 degree C, 15.6 hr at 20 degree C, 17.5 hr at 18 degree C, and 22.0 hr at 16 degree C (Supplemental Fig. 2). It may be useful in temperature-dependent studies to bring embryos to two different stages at the same time. Furthermore, we measured the tail and trunk length at each stage after stage 15 (Table 1).
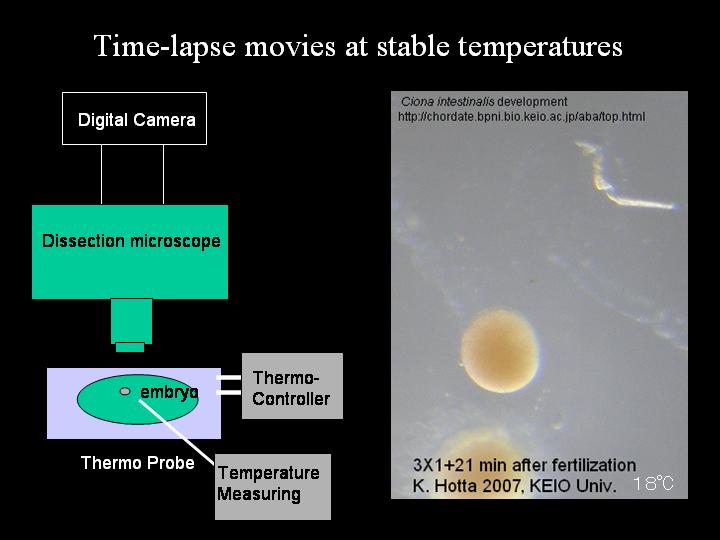
Ciona intestinalis adults were obtained from the Gamagohri or Yokohama areas, Japan. In this study, we used eggs in winter (February to March). Eggs and sperm were obtained surgically from the gonoduct. After insemination, eggs were dechorionated as described previously (Hotta et al., [1999]). Dechorionated fertilized eggs were maintained in agarose-coated dishes with Millipore-filtered seawater (MFSW) containing 50 micro g/ml streptomycin sulfate. To keep the temperature stable, we used a peltier-type incubator (CN-25B, Mitsubishi, Japan) without any vibration by a compressor to prevent embryo fusion. Embryos developed hatched larva approximately 18 hr after insemination.
By using a peltier-based thermo-controller ThermoPlate MATS-500 (Tokai Hit), which was placed on the microscope stage (Olympus SZX16), the temperature was stabilized and maintained. All temperature was measured by a ThermoProbe (IT-18, Bio Research Center), which was controlled by PowerLab (ADI instrument). The embryo placed on the thermo-plate was maintained for 20 hr to stabilize temperature at 16, 18, 20, and 22 degree centigrade, respectively, to acquire images. By using a digital camera (Olympus SP-350) mounted on the microscope, images were acquired every 3 min (Supplemental Movie 1).
Fixed embryos were prepared from fertilized eggs up to hatched larva. Embryos were fixed for 15 or 30 min at room temperature in 4% EM grade paraformaldehyde (nacalai tesque code 00380) in MOPS buffer (0.1 M 3-(N-Morpholino) propanesulfonic acid), adjusted to pH 7.5. Embryos were then washed three times with phosphate-buffered saline (PBS) and incubated in Alexa-546 phalloidin (Molecular Probes, Eugene OR) in PBS containing 0.01% Triton X-100 (PBST) either overnight at 4°C or at room temperature for 1-2 hr. Embryos were then rinsed for 3 min in PBS, attached to glass slide dishes, dehydrated through an isopropanol series, and finally cleared using Murray clear, a 2:1 mixture of benzyl benzoate and benzyl alcohol.
We collected images with a CLSM on a Zeiss LSM510 META with 40X oil objective. To reconstruct the 3D images, 100 cross-section images from top to bottom per sample were acquired. In total, over 3,500 Z-slice images corresponding to 35 developmental embryos were obtained, which allowed for overlapped developmental stages. The focus interval depends on the sample (from 0.5 to 1.2 micro m).
We thank deeply Nori Satoh for his overall support to publish this manuscript. We thank Patrick Lemaire, Jean-Stephane Joly, and Hiroki Nishida for their critical comments and discussion about the staging of C. intestinalis, Shigekazu Oda for technical support in time-lapse imaging, Minako Aoki, Masayuki Takahashi, Ryo Takano, and Yu Tanouchi for help on database construction, and P. Lemaire and J. S. Hwang for their critical reading of this manuscript. K.H. was supported by JSPS (18770207). This work was also supported by JST-BIRD and a grant-in-aid from MEXT.
At the same time as our study, a study aimed at the stagings of tailbud embryos was performed by J. S. Joly's group. A table with data compatible to our staging is shown in Supplemental Table 1.
| Additional Material |